Myriad Matrix™
Overview
Myriad Matrix™ is an engineered extracellular matrix (ECM) for soft tissue repair, reinforcement and complex wounds.
Myriad Matrix™ has been specifically designed to help maximize tissue repair while providing versatility and adaptability across a wide range of surgical applications.
Myriad Matrix™ is designed to be easily customizable for a wide range of anatomical sites and surgical needs and can be used in both implantation and dermal repair.
- Myriad Matrix™ is strong, soft, drapable, and conforming
- Myriad Matrix™ rehydrates quickly and is easy to cut, suture or staple
- Myriad Matrix™ helps to achieve surgical mastery in routine and challenging repair and reinforcement procedures
Myriad Matrix™ has been specifically designed for surgical soft tissue reconstruction and complex wounds.
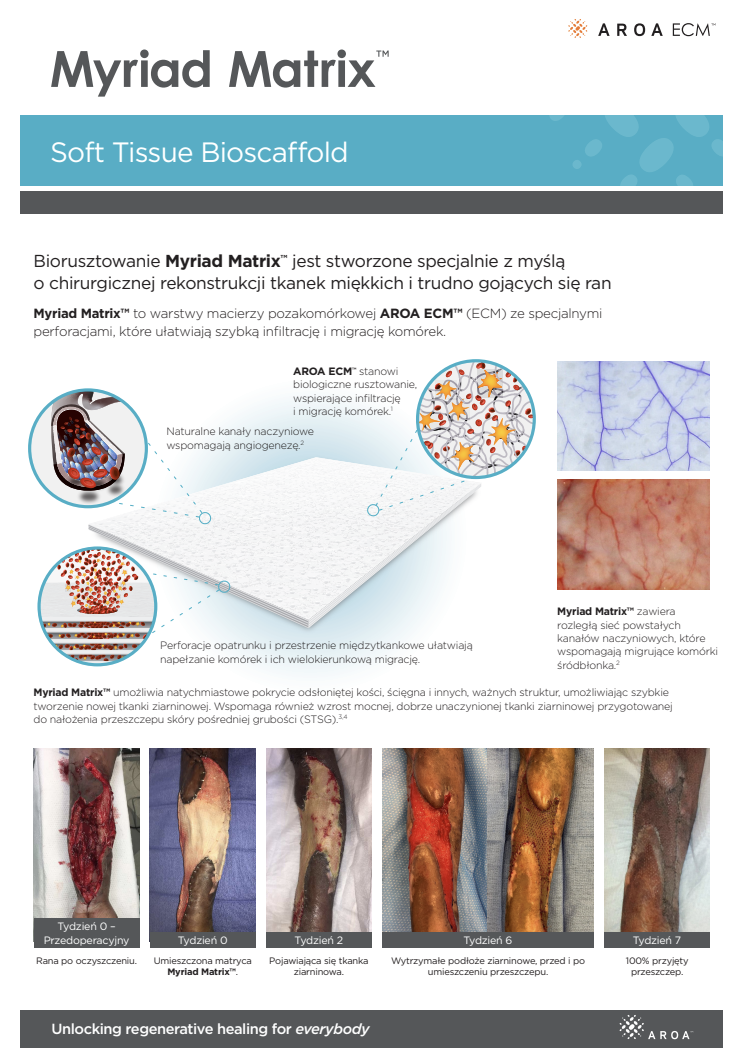
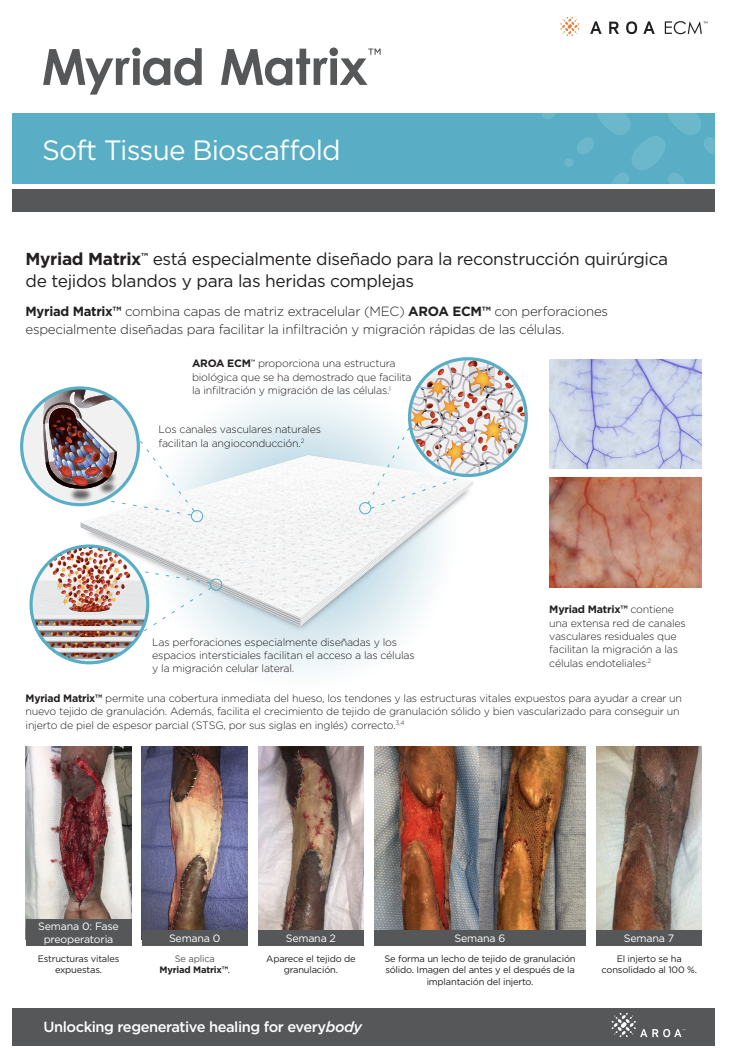
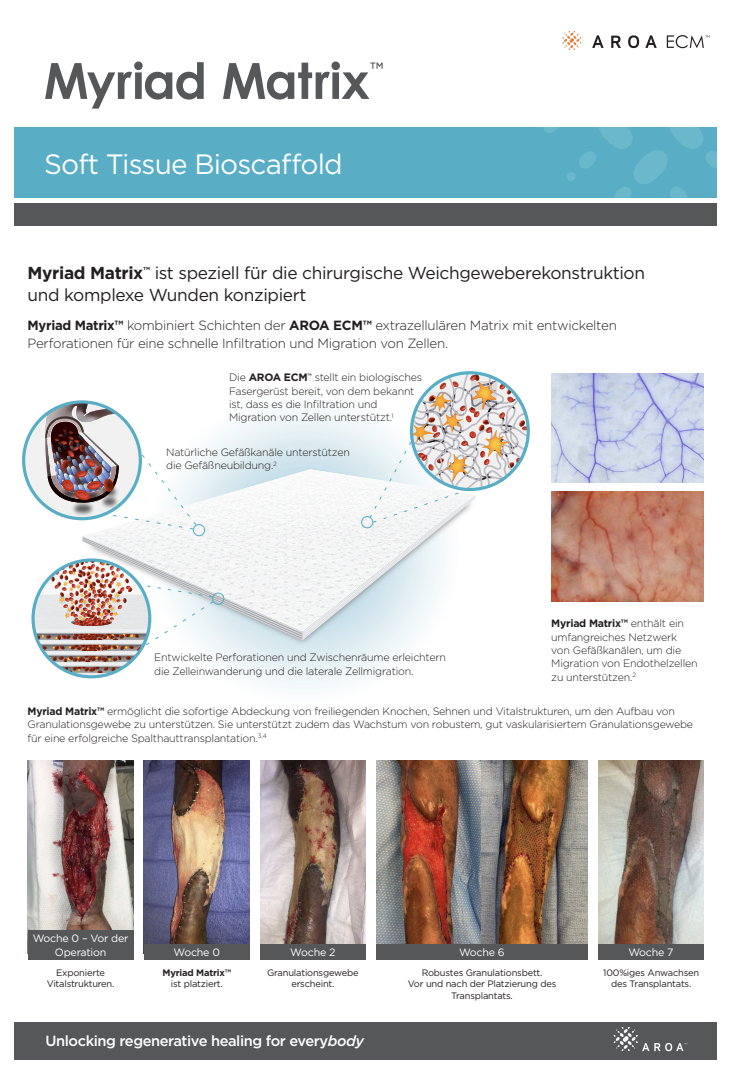
Myriad Matrix™ combines layers of AROA ECM™ extracellular matrix (ECM) with engineered perforations to facilitate rapid cell infiltration and migration in a wide range of anatomical sites and individual situations.
- Myriad Matrix™ is strong, soft, drapable, and conforming
- Myriad Matrix™ rehydrates quickly and is easy to cut, suture or staple
- Myriad Matrix™ helps to achieve surgical mastery in routine and more challenging dermal reconstructions and complex wounds

How It Works
Myriad Matrix™ devices contain the natural porous structure of AROA ECM™, engineered with interstitial perforations to enable cell infiltration to facilitate rapid healing.
Fast absorption of blood & cells accelerates host cell infiltration
The rapid absorption of blood and blood components forms a reservoir of biologically important cells and cell components to help kick-start the tissue repair process.(1-4) Fibroblast, endothelial and immune cells infiltrate the entire matrix, build new tissue and over time Myriad Matrix™ is completely remodeled by the patient’s own tissue.(3, 4)
Delivers biology known to be important in healing
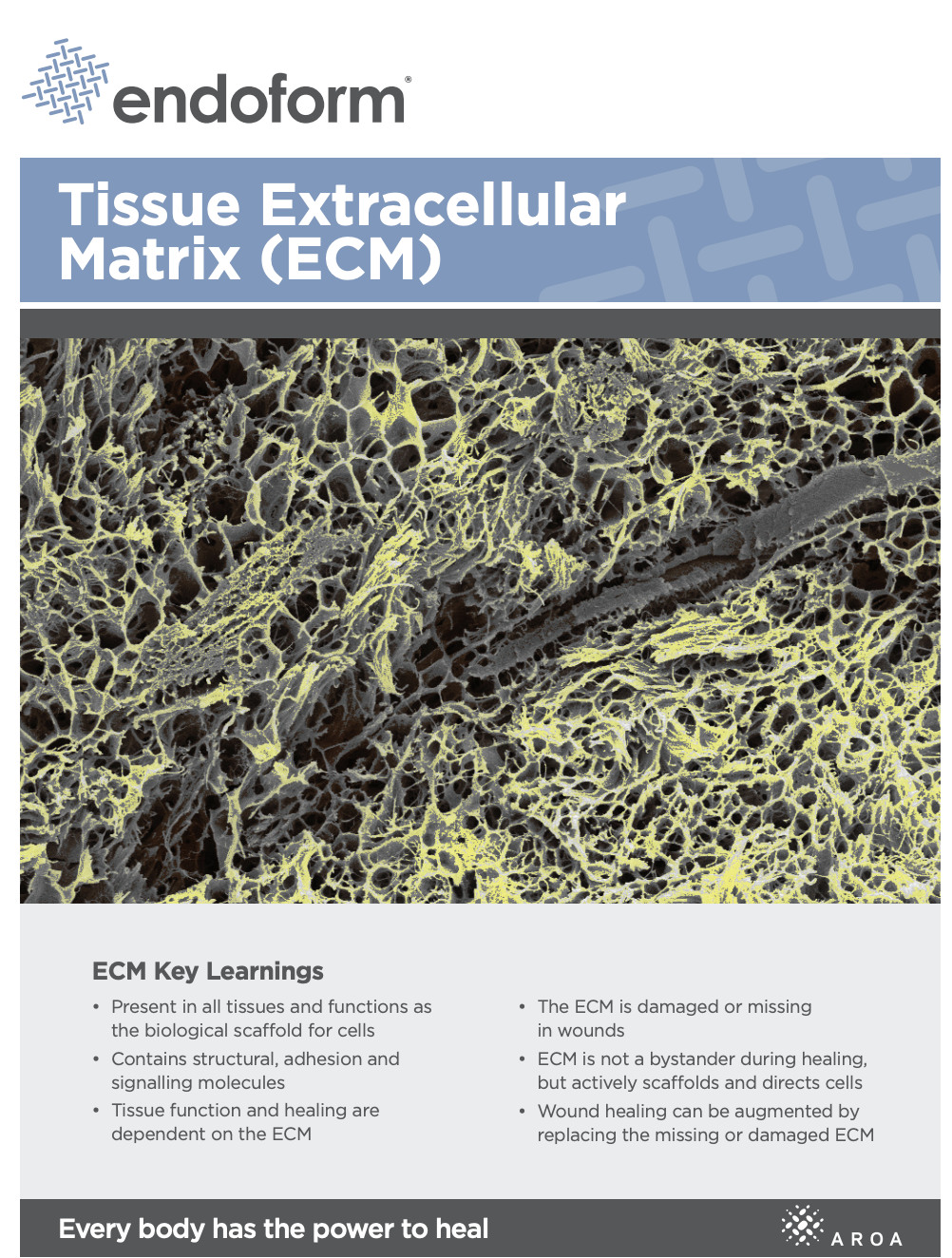
Myriad Matrix™ retains the authentic structure and complexity of natural tissue ECM and provides biological cues to aid the repair process.(5) Myriad Matrix™ contains more than 150 ECM proteins, including collagen and other secondary molecules that exist in tissue and aid the healing process.(6)
Contains residual vascular channels
Angioconduction is the structural effect of vascular channels on endothelial cells to support blood vessel development. Myriad Matrix™ promotes angioconduction via the residual vascular channels within the Aroa ECM™ source biomaterial, helping to facilitate the rapid establishment of a dense capillary network.(7, 8)

1. G. A. Bohn and A. E. Chaffin: Extracellular matrix graft for reconstruction over exposed structures: a pilot case series. J Wound Care, 29(12), 742-749 (2020) doi:10.12968/jowc.2020.29.12.742 2. A. E. Chaffin and M. C. Buckley: Extracellular matrix graft for the surgical management of Hurley stage III hidradenitis suppurativa: a pilot case series. J Wound Care, 29(11), 624-630 (2020) doi:10.12968/jowc.2020.29.11.624 3. S. M. Irvine, J. Cayzer, E. M. Todd, S. Lun, E. W. Floden, L. Negron, J. N. Fisher, S. G. Dempsey, A. Alexander, M. C. Hill, A. O’Rouke, S. P. Gunningham, C. Knight, P. F. Davis, B. R. Ward and B. C. H. May: Quantification of in vitro and in vivo angiogenesis stimulated by ovine forestomach matrix biomaterial. Biomaterials, 32(27), 6351-61 (2011) doi:l10.1016/j.biomaterials.2011.05.040 4. N. Overbeck, G. M. Nagvajara, S. Ferzoco, B. C. H. May, A. Beierschmitt and S. Qi: In-vivo evaluation of a reinforced ovine biologic: a comparative study to available hernia mesh repair materials. Hernia, 10.1007/s10029-019-02119-z (2020) 5. S. Lun, S. M. Irvine, K. D. Johnson, N. J. Fisher, E. W. Floden, L. Negron, S. G. Dempsey, R. J. McLaughlin, M. Vasudevamurthy, B. R. Ward and B. C. May: A functional extracellular matrix biomaterial derived from ovine forestomach. Biomaterials, 31(16), 4517-29 (2010) doi:10.1016/j.biomaterials.2010.02.025 6. S. G. Dempsey, C. H. Miller, R. C. Hill, K. C. Hansen and B. C. H. May: Functional Insights from the Proteomic Inventory of Ovine Forestomach Matrix. J Proteome Res, 18(4), 1657-1668 (2019) doi:10.1021/acs.jproteome.8b00908 7. Smith MJ, Dempsey SG, Veale RW, et al. Further structural characterization of ovine forestomach matrix and multi-layered extracellular matrix composites for soft tissue repair. Journal of Biomaterials Applications. 2022;36(6):996-1010. doi:10.1177/08853282211045770 8. N. S. Greaves, S. A. Lqbal, J. Morris, B. Benatar, T. Alonso-Rasgado, M. Baguneid and A. Bayat: Acute cutaneous wounds treated with human decellularised dermis show enhanced angiogenesis during healing. PLoS One, 10(1), e0113209 (2015) doi:10.1371/journal.pone.0113209
Indications
Myriad Matrix™ is indicated for implantation to reinforce soft tissue where weakness exists in patients requiring soft tissue repair or reinforcement in plastic and reconstructive surgery, or for management of the following wounds:
- Partial and full thickness wounds
- Pressure ulcers
- Venous ulcers
- Diabetic ulcers
- Chronic vascular ulcers
- Tunneled/ undermined wounds
- Surgical wounds (donor sites, grafts, post Moh’s surgery, post laser surgery, podiatric, wound dehiscence)
- Trauma wounds (abrasions, lacerations, second-degree burns, and skin tears)
- Draining wounds

Clinical Evidence
ClearConference Proceedings
- Gujju, M., R. Reid, J. Dickerson, L. Rezvani and A. E. Chaffin (2024). Morselized Ovine Forestomach Matrix (OFM) for Treatment of Degloving Injury: A Case Study in Innovative Treatment of Complex Wounds. Symposium on Advanced Wound Care (Fall), Oct 02 – 05, Las Vegas, NV. Full-text available | View
- Lawlor, J. C., B. A. Bosque, C. Frampton, D. A. Young and P. Martyka (2024). A Large, Real-World, Prospective, Single-Arm Study Evaluating Outcomes Following Complex Lower Extremity Reconstruction with Ovine Forestomach Matrix Graft. Symposium on Advanced Wound Care (Fall), Oct 02 – 05, Las Vegas, NV.
- Joseph, R. and A. J. LaLama (2024). Successful Treatment of Systemic Sclerosis Caused Ulceration Utilizing Ovine Forestomach Matrix Graft Symposium on Advanced Wound Care (SAWC) – Spring, May 14-18,, Ft. Lauderdale, FL. Full-text available | View
- Lawlor, J. C., B. A. Bosque, D. A. Young, C. Frampton and P. Martyka (2024). A Large, Real-World, Prospective, Single-Arm Study Evaluating Outcomes Following Complex Lower Extremity Reconstruction with Ovine Forestomach Matrix Graft. Diabetic Foot Global Conference (DFCon), Nov 7-9, Anaheim, CA.
- Anandarajan, V., J. C. Choi, K. Wells and P. Kumar (2024). Extracellular Matrix for the Treatment of Anal Fistula: A Single Center Retrospective Review. American College of Colon and Rectal Surgeons (ASCRS), June 1-4, Baltimore, MD. Full-text available | View
- Nasseri, Y., M. Barnajian and K. La (2024). Comparative Efficacy of Pilonidal Sinus Reconstruction Incorporating an Extracellular Matrix Graft. American College of Colon and Rectal Surgeons (ASCRS), June 1-4, Baltimore, MD. Full-text available | View
- Reid, J., J. Dickerson and A. Chaffin (2024). Morselized Ovine Forestomach Matrix (OFM) for Limb Salvage: A Case Report on Innovative Reconstruction in Lower Extremity Pressure Injury. Symposium on Advanced Wound Care (SAWC) – Spring, May 14-18, Ft. Lauderdale, FL Full-text available | View
- Trinh, S., J. Dennis, P. Deville, P. Greiffenstein, J. P. Hunt, A. B. Marr, L. Stuke and A. A. Smith (2024). Ovine Forestomach Matrix Graft as Part of Aggressive Surgical Management in Pressure Ulcers in Young Trauma Cohort: A Pilot Retrospective Case Series. Symposium on Advanced Wound Care (SAWC) – Spring, May 14-18, Ft. Lauderdale, FL. Full-text available | View
- Mason, A. J., K. R. K., J. C. Haouilou and J. D. Rhodenizer (2024). Pyoderma Gangrenosum: A case study with multiple surgical debridements and application of ovine forestomach matrix graft as alternative to below knee amputation. American College of Foot and Ankle Surgeons, Jan 31-Feb 4, Tampa, FL. Full-text available | View
- Trinh, S. (2024). A Case Series of Ovine Forestomach Matrix to Accelerate Enterocutaneous Fistula Healing After Emergency Exploratory Laparotomy (Oral Presentation). John A. Boswick, M.D. Burn and Wound Healing Symposium, Jan 27 – Feb 1, Maui, HI.
- Rizzo, J. A. (2024). Filling the Void: Ovine ECM Grafts Support Closure of Large Volume Trauma Defects (Oral Presentation). John A. Boswick, M.D. Burn and Wound Healing Symposium, Jan 27 – Feb 1, Maui, HI.
- Desvigne, M. (2024). Using ECM Graft Placement To Stabilize Tissue And Modulate Inflammation In A Stage 4 Pressure Injury – An Extreme Case (Oral Presentation). John A. Boswick, M.D. Burn and Wound Healing Symposium, Jan 27 – Feb 1, Maui, HI.
- Dardano, A. N. (2024). Use of SPY™ Imaging (ICG) to evaluate Myriad Matrix™ integration in complex wounds: Early Experience (Oral Presentation). John A. Boswick, M.D. Burn and Wound Healing Symposium, Jan 27 – Feb 1, Maui, HI.
- Cormican, M. T. (2024). Ovine Forestomach Matrix in the Surgical Management of Volumetric Soft Tissue Defects: A Retrospective Case Series (Oral Presentation). John A. Boswick, M.D. Burn and Wound Healing Symposium, Jan 27 – Feb 1, Maui, HI.
- Chaffin, A. E. (2024). Surgical Reconstruction of Stage 3 and 4 Pressure Injuries with ECM Technology: A Consensus Algorithm (Oral Presentation). John A. Boswick, M.D. Burn and Wound Healing Symposium, Jan 27 – Feb 1, Maui, HI.
- Taarea, R., A. Florence and C. Castater (2024). Novel Use of Extracellular Matrix Graft in the Surgical Management of Open Abdomen. The Southeastern Surgical Congress Annual Meeting, Feb 3-6, Clearwater Beach, FL. Full-text available | View
- Chaffin, A. E. and M. Desvigne (2024). Interim Analysis of Stage 4 Pressure Injuries Treated Following Proposed Pressure Injury Treatment Protocol Using Decellularized Extracellular Matrix Graft National Pressure Injury Advisory Panel Conference, Feb 16-17, San Antonio, TX. Full-text available | View
- Melin, M., R. Kaufman, J. E. J. Geiger, I. Zilberman, N. Zolfaghari, H. Que, J. Longobardi, J. W. Dutch, J. Skurka, B. A. Bosque and S. G. Dowling (2023). Clinical Effectiveness of Ovine Forestomach Matrix Graft in Complex Lower Limb Reconstruction and Limb Salvage. Nurses Specialized in Wound, Ostomy and Continence Canada, May 03-07,2023. Ottawa, Ontario, Canada. Full-text available | View
- Melin, M., R. Kaufman, J. E. J. Geiger, I. Zilberman, N. Zolfaghari, H. Que, J. Longobardi, J. W. Dutch, J. Skurka, B. A. Bosque, S. G. Dowling and B. C. H. May (2023). Clinical Effectiveness of Ovine Forestomach Matrix Graft in Complex Lower Limb Reconstruction and Limb Salvage. European Wound Management Association, 03-05 May 2023. Milan, Italy Full-text available | View
- Chaffin, A. E. (2023). Pressure Injury Reconstruction Utilizing an Ovine Forestomach Matrix Graft. Symposium on Advanced Wound Care (SAWC), April 26-30, 2023. National Harbor, Maryland, USA. Full-text available | View
- Lawlor, J. C. (2023). Surgical Management of Complex Limb Salvage with Ovine Forestomach Matrix. Symposium on Advanced Wound Care (SAWC), April 26-30, 2023. National Harbor, Maryland, USA Full-text available | View
- Cormican, M. T., N. J. Creel, P. P. Rideout and W. M. Vassy (2023). Ovine Forestomach Matrix in the Surgical Management of Complex Volumetric Soft Tissue Defects: A Retrospective Case Series. Symposium on Advanced Wound Care (SAWC), April 26-30, 2023. National Harbor, Maryland, USA. Full-text available | View
- Chaffin, A. E. (2023). Surgical Intervention for Atypical Wounds (Oral Presentation). APWCA – Wound Week, Sep 20-24, Philadelphia, PA.
- Chaffin, A. E., S. S. Awad, J. D. Stern, C. T. Milne, S. G. Dowling, R. Sotomayor, E. A. Ayello, L. J. Feo Aguirre, B. Z. Khalaf, L. J. Gould and M. N. Desvigne (2023). Surgical Reconstruction of Stage 3 and 4 Pressure Injuries with ECM Technology: A Proposed Algorithm. APWCA – Wound Week, Sep 20-24, Philadelphia, PA. Full-text available | View
- Taarea, R., B. Bendixen, A. Florence and C. Castater (2023). Ovine Forestomach Matrix in the Surgical Management of Traumatic Open Abdominal Defects. Georgia Society of the American College of Surgeons Annual Meeting, Aug 11-16, Simoms Island, GA. Full-text available | View
- Short, T. (2023). An Initial Experience with an Ovine Forestomach Matrix Graft and Particulate for Partial and Full Thickness Wounds: A Retrospective Case Series (Oral Presentation). John A. Boswick, M.D. Burn and Wound Healing Symposium, Jan 21 – 26, Maui, HI.
- Deville, P., F. Lau and A. A. Smith (2023). Ovine Derived Extracellular Matrix as an Alternative for Chronic Wounds not Amendable to Flap or Graft Coverage (Oral Presentation). John A. Boswick, M.D. Burn and Wound Healing Symposium, Jan 21 – 26, Maui, HI.
- Chaffin, A. E. (2023). Augmenting Pressure Injury Flap Reconstruction with an Ovine Forestomach Matrix Graft: A Retrospective Case Series (Oral Presentation). John A. Boswick, M.D. Burn and Wound Healing Symposium, Jan 21 – 26, Maui, HI.
- Wolf, J. H. (2023). Surgical Management of Perianal Fistula Using an Ovine Forestomach Matrix Implant (Video Abstract). ASCRS Annual Scientific Meeting, June 3-6, Seattle, WA.
- Nasseri, Y., K. Oka, K. La, A. Solis, J. Cohen and M. Barnajian (2023). Prospective Registry Study Evaluating Ovine Forestomach Matrix Graft As Part Of Pilonidal Sinus Flap Reconstruction – Interim Analysis. ASCRS Annual Scientific Meeting, June 3-6, Seattle, WA. Full-text available | View
- Chaffin, A. E. and M. N. Desvigne (2023). Pressure Injury Reconstruction Utilizing an Ovine Forestomach Matrix Graft. National Pressure Injury Advisory Panel, 17-18 March, 2023. San Diego, California, USA. Full-text available | View
- Taarea, R., J. J. Donahue and C. Castater (2022). Ovine Forestomach Matrix in the Surgical Management of Complex and Contaminated Soft Tissue Trauma. Symposium for Advanced Wound Care – Fall, November 2-5, Las Vegas, NV. Full-text available | View
- Register, A. (2022). A retrospective review of an extracellular matrix used to treat complex chronic wounds. Symposium on Advanced Wound Care – Spring 2022, April 6-10, 2022, Phoenix, AZ. Full-text available | View
- Melin, M., R. Kaufman, J. E. J. Geiger and B. A. Bosque (2022). Ovine Forestomach Matrix in the Surgical Management of Complex Lower Extremity Soft Tissue Defects : A Retrospective Multi-Center Case Series. American Professional Wound Care Association, Wound Week, 2022 (February 24-27, 2022) Loews Philadelphia Hotel, Philadelphia, PA. Full-text available | View
- Melin, M. M., R. Kaufman, J. E. J. Geiger, I. Zilberman, N. Zolfaghari, H. Que, J. Longoardi, W. Dutch, J. Skurka, B. A. Bosque and S. G. Dowling (2022). Clinical Effectiveness of Ovine Forestomach Matrix Graft in Complex Lower Limb Reconstruction and Limb Salvage. Symposium on Advanced Wound Care – Spring, April 6-10, 2022, Phoenix, AZ. Full-text available | View
- Melin, M. (2022). Clinical Effectiveness of Ovine Forestomach Matrix Graft in Complex Lower Limb Reconstruction and Limb Salvage (Oral Presentation). John A. Boswick Burn and Wound Care Symposium January 21-26, 2022, Wailea Beach Resort – Marriott – Wailea – Maui, Hawaii.
- Hsu, A. T.-W., K. Schlidt, C. R. D’Adamo, B. A. Bosque, S. G. Dowling and J. H. Wolf (2022). Successful outcomes in anal fistula treatment using ovine forestomach matrix implant technique. ASCRS Annual Scientific Meeting, April 30 – May 4, Tampa, FL. Full-text available | View
- Howie, Q., J. Longobardi, M. M. Melin, R. Kaufman, J. J. E. Geiger, I. Zilberman, J. W. Dutch, J. Skurka, B. A. Bosque and S. G. Dowling (2022). Clinical Effectiveness of Ovine Forestomach Matrix Graft in Complex Lower Limb Reconstruction and Limb Salvage. Diabetic Global Foot Conference, September 29th-October 1st, Los Angeles, CA. Full-text available | View
- Kokal, W. and P. Price (2022). A Case Report of the Treatment of Pemphigus Vulgaris Utilizing an Extracellular Matrix Graft (Oral Presentation). John A. Boswick Burn and Wound Care Symposium January 21-26, 2022, Wailea Beach Resort – Marriott – Wailea – Maui, Hawaii.
- LaLama, A. J. (2022). Ovine Forestomach Matrix in the Surgical Management of Scleroderma Skin Ulcer. Symposium for Advanced Wound Care – Fall, November 2-5, Las Vegas, NV. Full-text available | View
- Geiger, J. E. J., R. Kaufman, I. Zilberman, N. Zolfaghari, W. Dutch and B. A. Bosque (2022). Continuity of Care: Retrospective Review of Ovine Forestomach Matrix Efficacy Across All Phases of Wound Healing. Symposium on Advanced Wound Care – Spring 2022, April 6-10, 2022, Phoenix, AZ. Full-text available | View
- Desrosiers III, A. (2022). Postsurgical Pyoderma Gangrenosum in Bilateral Breast Reduction Surgery. Innovations in Wound Healing, Dec 04 – 07, 2022. Florida, USA.
- Chaffin, A. E., J. Christopher and B. Salah (2022). A retrospective review of soft tissue reconstruction in volumetric soft tissue defects. Symposium on Advanced Wound Care – Spring 2022, April 6-10, 2022 Phoenix, AZ. Full-text available | View
- Chaffin, A. E., M. Melin and L. Mohan (2022). Novel treatment algorithm of Pyoderma Gangrenosum with Hypochlorous acid and Ovine Forestomach Matrix; Can the dogma of pathergy be altered? Innovations in Wound Healing, Dec 04 – 07, 2022. Florida, USA.
- Chaffin, A. E. (2022). Morselized Extracellular Matrix Graft to Improve Split Thickness Skin Graft Outcomes: A Retrospective Case Series (Oral Presentation). John A. Boswick Burn and Wound Care Symposium January 21-26, 2022, Wailea Beach Resort – Marriott – Wailea – Maui, Hawaii.
- Chaffin, A. E. (2022). Full Thickness Wound Reconstruction After Wide Excision of Recurrent Plantar Fibromatosis. Symposium for Advanced Wound Care – Fall, November 2-5, Las Vegas, NV.Symposium for Advanced Wound Care – Fall, November 2-5, Las Vegas, NV. Full-text available | View
- Chaffin, A. E. (2022). A surgical technique for flap reconstruction of pilonidal sinus using extracellular matrix graft (oral presentation). World Union of Wound Healing Societies, 1-5 March 2022, Abu Dhabi.
- Canfield, A. and R. Kemp (2022). Ovine Forestomach Matrix in the Management of a Complicated Abdominal Surgical Dehiscence. Symposium for Advanced Wound Care – Fall, November 2-5, Las Vegas, NV. Full-text available | View
- Holloway, B. (2021). Minimally Invasive Closure for Recurrent Pilonidal Sinus Using Extracellular Matrix Graft: A Case Report. Symposium on Advanced Wound Care – Fall, 2021 (October 29-31), Las Vegas, NV. Full-text available | View
- Geiger, A. J., B. A. Bosque and R. Kaufman (2021). Clinical Effectiveness of Ovine Forestomach Matrix for Coverage of Exposed Vital Structures in Complex Diabetic Foot Ulcer Reconstructions. DFCon – Diabetic Global Foot Conference, October 21 – 23, 2021. San Francisco, California. Full-text available | View
- Chaffin, A. E. and J. Christopher (2021). A retrospective review of the early experience with a morselized extracellular matrix. Symposium on Advanced Wound Care – Fall, 2021 (October 29-31), Las Vegas, NV. Full-text available | View
- Chaffin, A. E. (2021). Flap Reconstruction of Pilonidal Sinus Using Extracellular Matrix Graft: A Pilot Case Series. Symposium on Advanced Wound Care (SAWC) – Spring, Virtual. Full-text available | View
- Chaffin, A. E. (2021). A Surgical Technique for Flap Reconstruction of Pilonidal Sinus Using Extracellular Matrix Graft (Oral Presentation). 44th Annual John A. Boswick Burn & Wound Symposium, Maui, HI.
- Wahab, N., Lapucha, M., and C. Chauhan (2020). Ovine Forestomach Matrix is Efficacious in the Closure of Significant Post Surgical Undermining. Symposium on Advanced Wound Care – Spring, Virtual Conference. Full-text available | View
- Geiger, J. E. J. and J. Skwarczyk (2020). Extracellular matrix-based regeneration over exposed bone and tendon: A prospective case series. DFCon 20 Conference, Oct 9-10 2020, Virtual. Full-text available | View
- Chaffin, A. E., and M. Buckley (2020). Clinical Evaluation of an Extracellular Matrix Graft for the Surgical Management of Hurley Grade III Hidradenitis Suppurativa. Symposium of Advanced Wound Care, Virtual Conference. Full-text available | View
- Chaffin, A. E. (2020). Pilonidal Cystectomy Using Extracellular Matrix Graft. Symposium on Advanced Wound Care – Fall, November 4-6 2020, Virtual. Full-text available | View
- Ferreras, D. T. and R. L. Crump (2019). Use of a High-Density ECM for the Management of Deep Diabetic Foot Ulcers: A Case Series. Symposium for Advanced Wound Care – Fall, Las Vegas, NV. Full-text available | View
- Chaffin, A. E., A. M. Aballay, G. A. Bohn, P. M. Glat, M. N. Desvigne and B. C. H. May (2019). Multi-Centre Clinical Evaluation of a Cell Conductive Extracellular Matrix Surgical Mesh in Plastics and Reconstructive Surgery – A Case Series. 41st Annual Boswick Burn & Wound Symposium, Wailea Beach, Maui, HI. Full-text available | View
- Chaffin, A. E., A. M. Aballay, G. A. Bohn, P. M. Glat, M. N. Desvigne and B. C. May (2019). Multi-Centre Clinical Evaluation of a Cell Conductive Extracellular Matrix Surgical Mesh in Plastics and Reconstructive Surgery – A Case Series. SESPRS 62nd Annual Scientific Meeting, Naples, Florida. Full-text available | View
- Chaffin, A. E. and G. Bohn (2019). Clinical Evaluation of an Extracellular Matrix Surgical Graft for Reconstructive Surgery over Exposed Bone or Tendon. Symposium for Advanced Wound Care – Fall, Las Vegas, NV. Full-text available | View
Peer-Reviewed Publications
- Lawlor, J. C., B. A. Bosque, C. Frampton, D. A. Young and P. Martyka (2024). “Limb Salvage via Surgical Soft-tissue Reconstruction With Ovine Forestomach Matrix Grafts: A Prospective Study.” PRS Global Open 12(12): e6406 Full-text available | View
- Mosquera, C., S. Kang and C. A. Ramirez (2024). “Applications of Extracellular Matrix Biomaterial in Tongue Reconstruction.” J Craniofac Surg 35(7): e664-e666 Full-text available | View
- Su, T. (2024). “Initial experience with ovine forestomach matrix graft for glossectomy defect reconstruction: A case series.” Oral and Maxillofacial Surgery Cases 10(3): 100369. Full-text available | View
- Taarea, R., A. Florence, B. Bendixen and C. A. Castater (2024). “Early Experience with Ovine Forestomach Matrix for the Reconstruction of Abdominal Defects following Emergency Open Abdomen Surgery at a Level 2 Trauma Center.” Trauma Cases Rev 10(1): 102. Full-text available | View
- Hsu, A., K. Schlidt, C. R. D’Adamo, B. A. Bosque, S. G. Dowling and J. H. Wolf (2023). “Surgical management of perianal fistula using an ovine forestomach matrix implant.” Techniques in Coloproctology Full-text available | View
- Duplechain, A. B., B. A. Bosque, C. W. Fligor and A. E. Chaffin (2023). “Soft Tissue Reconstruction With Ovine Forestomach Matrix After Wide Excision of Plantar Fibromatosis.” ePlasty 2023(23): e20. Full-text available | View
- Fragoso, N. M., R. Masson, T. J. Gillenwater, V. Y. Shi and J. L. Hsiao (2023). “Emerging Treatments and the Clinical Trial Landscape for Hidradenitis Suppurativa—Part II: Procedural and Wound Care Therapies.” Dermatology and Therapy 13(8): 1699-1720. Full-text available | View
- Cormican, M. T., N. J. Creel, B. A. Bosque, S. G. Dowling, P. P. Rideout and W. M. Vassy (2023). “Ovine Forestomach Matrix in the Surgical Management of Complex Volumetric Soft Tissue Defects: A Retrospective Pilot Case Series.” ePlasty 23: e66 Full-text available | View
- Bosque, B. A., S. G. Dowling, B. C. H. May, R. Kaufman, I. Zilberman, N. Zolfaghari, H. Que, J. Longobardi, J. Skurka, J. E. Geiger and M. M. Melin (2023). “Ovine Forestomach Matrix in the Surgical Management of Complex Lower-Extremity Soft-Tissue Defects.” J Am Podiatr Med Assoc 113(3): 22-081. Full-text available | View
- Hamaguchi, R., T. L. Wearda, A. S. Volk, K. M. Kramer, A. B. Kimball, A. E. Chaffin and D. P. Orgill (2022). “Surgical Management of Hidradenitis Suppurativa: A Two-Center Retrospective Study.” Plast Reconstr Surg 150(5): 1115-1127. Full-text available | View
- Chaffin, A. E., S. G. Dowling, M. S. Kosyk and B. A. Bosque (2021). “Surgical reconstruction of pilonidal sinus disease with concomitant extracellular matrix graft placement: a case series.” J Wound Care 30(Sup7): S28-S34. Full-text available | View
- Chaffin, A. E. and M. C. Buckley (2020). “Extracellular matrix graft for the surgical management of Hurley stage III hidradenitis suppurativa: a pilot case series.” J Wound Care 29(11): 624-630. Full-text available | View
- Desvigne, M. N., K. Bauer, K. Holifield, K. Day, D. Gilmore and A. L. Wardman (2020). “Case Report: Surgical Closure of Chronic Soft Tissue Defects Using Extracellular Matrix Graft Augmented Tissue Flaps.” Frontiers in Surgery 7(173): 559450 Full-text available | View
- Bohn, G. A. (2020). “Using Ovine Extracellular Matrix in Difficult to Close Excisions of Common Skin Cancer: an Evolving New Technique.” Surg Technol Int 37: 49-53. Full-text available | View
- Bohn, G. A. and A. E. Chaffin (2020). “Extracellular matrix graft for reconstruction over exposed structures: a pilot case series.” J Wound Care 29(12): 742-749. Full-text available | View
- Simcock, J. and B. C. May (2013). “Ovine forestomach matrix as a substrate for single-stage split-thickness graft reconstruction.” Eplasty 13: e58. Full-text available | View
Product Resources
Video Library
A Surgical Technique for Flap Reconstruction of Pilonidal Sinus Using Extracellular Matrix Graft
Watch VideoDownloadable Documents
Reimbursement
Aroa Biosurgery is committed to offering a suite of advanced extracellular matrix (ECM) products that not only spans the different sites of care, but also offers the AROA Continuum of Care allowing you to select the product based on your healing objectives and application setting.
This page is dedicated to reimbursement information and support for our products. Should you wish to speak to one of our specialist reimbursement support team, please contact us on:
- reimbursement@aroa.com
- 1800 AROA ECM (1-800-2762-326)
- Fax: +1 877-775-3157
Should you require additional or more comprehensive information, please find it available on the Centers for Medicare and Medicaid Services website.
Product availability may vary by country, Please check with your local sales representative or distributor for more information.